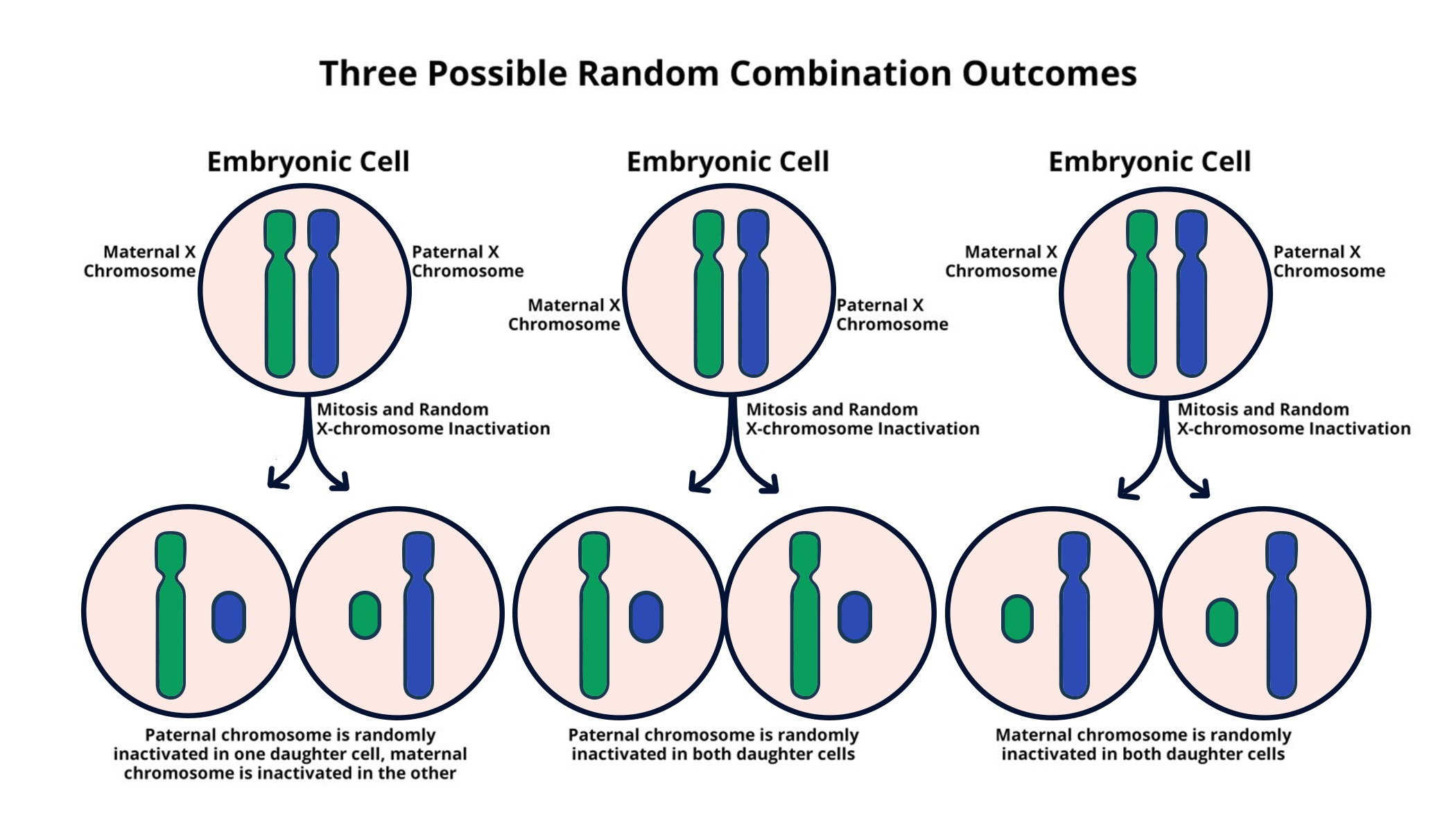
X-chromosome inactivation is a fascinating biological process that ensures balance in genetic expression between males and females. In females, with two X chromosomes, one chromosome is selectively silenced to prevent an overload of gene products, a phenomenon crucial for normal development. Understanding how this chromosomal silencing occurs is pivotal, especially in the context of genetic diseases linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Researchers like Jeannie T. Lee are unveiling the roles played by the Xist RNA molecule in orchestrating this intricate mechanism, which could revolutionize therapies for these conditions. As advancements continue, the implications for treating other genetic diseases tied to the X chromosome are increasingly promising.
The process of silencing one of the X chromosomes, known as X-inactivation, highlights a remarkable aspect of genetic regulation in mammals. This phenomenon particularly affects females, who carry a pair of X chromosomes, requiring a method to prevent gene dosage imbalance. The study of chromosomal silencing mechanisms and their roles in various genetic disorders opens new doors for potential treatments, particularly for disorders such as Fragile X and Rett syndromes. As scientists delve deeper, they uncover the pivotal contributions of molecules like Xist RNA, which are essential for achieving proper X-chromosome functionality. With ongoing research, the path toward innovative therapies for related genetic diseases appears increasingly attainable.
Understanding X-Chromosome Inactivation
X-chromosome inactivation is a crucial biological process that occurs in female mammals to balance the gene dosage between genders. Since females possess two copies of the X chromosome, one copy is inactivated in each cell at random, effectively equalizing gene expression with males, who only have one X chromosome. This process is crucial for proper cellular function and development, as it prevents the potential overexpression of genes that can lead to developmental issues and genetic disorders. The mechanism behind X-inactivation is complex and involves various molecules that modulate the chromosomal environment, often characterized as a gelatinous substance used to facilitate chromosomal silencing.
The significance of X-chromosome inactivation extends beyond basic biology; it holds immense implications for treating genetic diseases like Fragile X Syndrome and Rett Syndrome. In these disorders, mutations on the X chromosome result in cognitive and developmental challenges. Understanding how the X chromosome is inactivated not only sheds light on normal cellular function but also opens up pathways for therapies aimed at ‘unsilencing’ genes that could restore normal function. This could potentially provide effective treatments for conditions that predominantly affect females due to their reliance on proper X-linked gene expression.
The Role of Xist RNA in Chromosomal Silencing
Xist RNA plays a pivotal role in the process of X-chromosome inactivation by instigating a series of molecular interactions that fundamentally alter the chromosomal landscape. When the Xist gene is activated, it produces a long non-coding RNA that binds to the X chromosome it originates from. This binding triggers a cascade of events, leading to the recruitment of proteins and modifications that render the chromosome silenced. This innovative mechanism ensures that one of the two X chromosomes in females is effectively ‘turned off’, a phenomenon directly labeled as chromosomal silencing. Understanding the function of Xist RNA not only enhances our comprehension of gene regulation but also identifies potential targets for therapeutic interventions in X-linked disorders.
The manipulation of Xist RNA presents an exciting avenue in the treatment of genetic diseases associated with the X chromosome. By exploring the pathways that Xist engages during the inactivation process, researchers aim to develop strategies to reverse this silencing in cases where it leads to disease. For instance, therapies targeting Xist could potentially allow the functional copy of a mutated gene to be expressed, thereby alleviating symptoms in diseases such as Fragile X Syndrome. As these developments progress, they can lead to innovative Rett Syndrome therapy methodologies that could offer significant hope for improved quality of life in affected individuals.
Innovations in Treating Fragile X Syndrome and Rett Syndrome
Recent advancements in understanding the biology of the X chromosome have opened new pathways for the treatment of genetic disorders, specifically Fragile X Syndrome and Rett Syndrome. Research conducted by Jeannie Lee’s lab at Harvard Medical School has focused on deciphering the mechanisms behind X-chromosome inactivation. The novel insights gained from this research underscore the potential for developing therapies that could unsilence X-linked genes, thereby offering a transformative approach for individuals affected by these conditions. Therapy efforts aim at harnessing the knowledge of chromosomal silencing and utilizing it to reinstate the function of genes that are otherwise rendered inactive due to mutations.
The clinical implications of these findings are profound, as they suggest a future where targeted treatments could effectively mitigate the symptoms associated with these neurodevelopmental disorders. The Lee lab’s promise of moving towards clinical trials holds optimism for many families grappling with the consequences of Fragile X and Rett Syndromes. By leveraging the principles of genetic intervention and chromosomal science, these therapies could potentially re-enable the expression of crucial genes, suggesting a significant step toward effective management and treatment of these lifelong conditions.
The Implications of Chromosomal Jell-O in Medical Research
The analogy of ‘chromosomal Jell-O’ developed by Lee and colleagues effectively captures the complexity of chromosomal architecture and its significance in genetic functioning. This gelatinous environment surrounding the chromosomes is not merely a passive structure; it plays a dynamic role in facilitating gene accessibility and function. Understanding how this chromosomal Jell-O interacts during the processes of X-inactivation and gene silencing is crucial for medical research, particularly when addressing genetic ailments linked to the X chromosome. This foundational knowledge assists scientists in formulating strategies to manipulate the chromosomal dynamics to restore gene function in a targeted manner.
This concept is especially relevant in the context of personalized medicine, as research into the physical properties of chromosomal Jell-O may yield insights into tailored therapeutic approaches for individual patients with genetic disorders. By determining how variations in this chromosomal environment affect gene expression and silencing, researchers can devise specific treatment plans that could successfully unsilence problematic genes, leading to more effective management options for conditions such as Fragile X Syndrome and Rett Syndrome. Therefore, continued exploration of this gelatinous coating around the chromosomes demonstrates not only a fundamental biological insight but a powerful tool for future healthcare innovations.
Genetic Diseases Linked to X Chromosome: An Overview
X-linked genetic diseases predominantly affect males due to their single X chromosome, but females can also be severely impacted when mutations occur on their X chromosomes. Conditions such as Fragile X Syndrome and Rett Syndrome illustrate the direct consequences of detrimental mutations located on the X chromosome. The intricate mechanisms of X-chromosome inactivation further complicate the clinical manifestations of such genetic disorders, as the inactivation can obscure the presence of a healthy allele in females, prompting the need for innovative treatments that can address the silencing of genes. Medical research in this area continues to rapidly evolve, focusing on possible therapies aimed at activating these silent genes.
Understanding the genetic basis of diseases linked to the X chromosome emphasizes the importance of studying mechanisms such as chromosomal silencing and the roles of specific molecules like Xist. By unraveling these genetic intricacies, researchers are positioned to pioneer new therapies that can reverse the effects of X-linked disorders. The urgency to address these diseases has gained momentum as the understanding of their underlying biology enhances, paving the way for prospective treatments that not only mitigate symptoms but potentially cure the genetic conditions at their core.
The Future of Genetic Research: Insights from X-Chromosome Studies
The groundbreaking work surrounding X-chromosome inactivation and the surrounding biological mechanisms symbolizes the future of genetic research. As insights from studies focusing on the X chromosome and its atypical behaviors continue to unfold, the medical community anticipates advancements in treating various genetic disorders linked to this chromosome. The potential of restoring gene function through targeted therapies presents a beacon of hope for individuals afflicted by conditions such as Fragile X Syndrome and Rett Syndrome. This push towards translating basic research into clinical practice represents a critical evolution in the approach to treating genetic diseases.
Furthermore, the interdisciplinary nature of genetic studies, combining molecular biology, genetics, and therapeutic innovations, fosters a collaborative atmosphere that accelerates discovery and application. The continued exploration of Xist RNA and its role as a key regulator in X-chromosome inactivation will systematically influence therapeutic strategies, potentially resulting in breakthroughs that could lead to effective treatments for various disorders beyond just those associated with the X chromosome. By responsibly harnessing the knowledge of chromosomal dynamics, researchers are not just answering longstanding scientific questions but actively working towards creating tangible solutions for genetic diseases.
Challenges in Genomic Therapies for X-Linked Disorders
Despite significant advancements in the understanding of genetic diseases linked to the X chromosome, challenges remain in developing effective genomic therapies. The complexity of chromosomal silencing mechanisms, particularly those surrounding X-chromosome inactivation, poses scientific hurdles that require innovative solutions. Therapeutic approaches that aim to manipulate the inactivation process must account for the delicate balance of gene expression, ensuring that previously silenced genes can be expressed without inadvertently disrupting the function of other essential genes. This precision is paramount for minimizing potential side effects, particularly in therapies targeting conditions such as Fragile X Syndrome and Rett Syndrome.
Moreover, the path from laboratory discoveries to clinical applications can be fraught with regulatory challenges and the need for comprehensive safety profiles in new therapeutic candidates. As researchers like Jeannie Lee advance their studies toward clinical trial phases, it is essential to ensure that the therapies not only effectively restore gene function but also maintain the overall genomic integrity of the patient. Ensuring rigorous testing and research integrity will be critical in ushering these promising treatments from lab benches to patients’ bedsides.
Genomic Interventions and Their Ethical Considerations
As the field of genetic therapies progresses, it is essential to address the ethical considerations surrounding genomic interventions, particularly those related to X-linked disorders. With the capability to manipulate gene expression and chromosomal features comes the responsibility to address the potential implications these therapies may carry with respect to genetic identity, consent, and the long-term effects on individuals and future generations. The concept of ‘unsilencing’ genes to treat conditions like Fragile X Syndrome and Rett Syndrome raises questions about the extent to which we can or should alter genetic expressions, and the possible societal ramifications of such interventions.
Ethics play an integral role in guiding research and therapeutic development to ensure that interventions respect patients’ autonomy and promote equity in access to innovative treatments. It is critical that discussions surrounding genomic therapies include diverse perspectives, acknowledging cultural, ethical, and social dimensions that impact how such therapies are viewed and implemented. As research moves forward, collaborative efforts between scientists, ethicists, and health policymakers are essential to navigate these complex issues, ensuring that advancements in treating genetic diseases are both scientifically sound and ethically responsible.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important?
X-chromosome inactivation is a biological process that occurs in females where one of the two X chromosomes is silenced to prevent an excess of gene products. This process is crucial for maintaining genetic balance between males and females, as males only have one X chromosome. Understanding this mechanism is vital for addressing genetic diseases linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, as it can lead to potential treatments.
How does the Xist RNA molecule contribute to X-chromosome inactivation?
The Xist RNA molecule plays a key role in X-chromosome inactivation by coating the X chromosome, facilitating its silencing. Once Xist binds to the X chromosome, it modifies the surrounding chromosomal environment, making it more flexible and allowing other molecules to assist in the inactivation process. This enables the inactivated X chromosome to remain inactive, which is essential for the biological balance and for potential therapies targeting X-linked genetic disorders.
What are the implications of X-chromosome inactivation for Fragile X Syndrome treatment?
The study of X-chromosome inactivation has significant implications for Fragile X Syndrome treatment. By developing methods to unsilence the inactivated X chromosome, researchers aim to restore the function of healthy genes that are silenced due to mutations. This approach could provide new therapeutic avenues for individuals with Fragile X Syndrome by potentially alleviating symptoms and improving cognitive function.
Can X-chromosome inactivation lead to therapies for genetic diseases?
Yes, insights gained from understanding X-chromosome inactivation can lead to therapies for genetic diseases linked to the X chromosome. The potential to unsilence inactivated X chromosomes provides a pathway for treating conditions like Rett Syndrome and Fragile X Syndrome by reactivating functional genes that are otherwise inaccessible due to chromosomal silencing.
What challenges remain in understanding X-chromosome inactivation?
Despite advancements in understanding X-chromosome inactivation, several challenges remain. Researchers continue to investigate how exactly the process can be manipulated to free inactivated X chromosomes without disrupting healthy gene function. Additionally, the specifics of why only certain genes are affected by unsilencing remain unclear, presenting further avenues for research into genetic diseases linked to the X chromosome.
| Key Points | Details |
|---|---|
| X-Chromosome Inactivation | Occurs in females to prevent overexpression of genes on the X chromosome. |
| Role of Xist | Xist RNA changes the properties of surrounding chromosomal material, leading to the silencing of one X chromosome. |
| Challenges | Understanding the mechanism of X-inactivation has taken decades of research and is crucial for therapy development. |
| Therapeutic Potential | Treatments may unsilence X-linked genes, potentially curing disorders like Fragile X and Rett syndromes. |
| Future Research | Optimizing unsilencing techniques and conducting safety studies prior to clinical trials. |
Summary
X-chromosome inactivation is a critical biological process that balances gene expression between sexes, with significant implications for understanding genetic diseases. Research led by Jeannie T. Lee has unraveled the complex mechanisms involved in this process, particularly focusing on how Xist RNA interacts with chromosomal material to silence genes. The groundbreaking findings not only illuminate fundamental cellular biology but also pave the way for potential therapies that could reverse the effects of mutations linked to conditions such as Fragile X and Rett syndromes, offering hope for those affected by these genetic disorders.